MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Compressibility of real gas will be less than ideal gas: temperature of gas, Boyle's temperature of gas
(a) At very high pressure, when
(b) At very high pressure when,
(c) At low pressure, when
(d) At low pressure, when
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main/Advance
IMPORTANT
The average velocity of gas molecules is . Calculate its r.m.s. velocity at the same temperature.
EASY
JEE Main/Advance
IMPORTANT
According to kinetic theory of gases
EASY
JEE Main/Advance
IMPORTANT
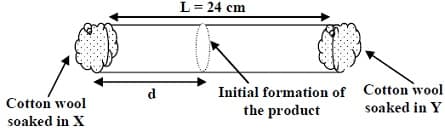
The value of $d$ in $\mathrm{cm}$ (shown in the figure), as estimated from Graham's law, is:
EASY
JEE Main/Advance
IMPORTANT
According to the kinetic theory of gases, between two successive collisions, a gas molecule travels
MEDIUM
JEE Main/Advance
IMPORTANT
What volume of hydrogen gas, at and pressure will be consumed in obtaining of elemental boron (atomic mass ) from the reduction of boron trichloride by hydrogen?
EASY
JEE Main/Advance
IMPORTANT
As the temperature is raised from to , the average kinetic energy of neon atoms changes by a factor :
MEDIUM
JEE Main/Advance
IMPORTANT
Which one of the following statements is not true about the effect of an increase in the temperature on the distribution of the molecular speeds in a gas?
EASY
JEE Main/Advance
IMPORTANT
Equal weights of methane and oxygen are mixed in an empty container at . The fraction of the total pressure exerted by oxygen is:
