Concentrated is converted into oxide by roasting.
How impurities like sulphur and phosphorous are removed in this process?

Important Questions on Production of Metals
Alumina is mixed with Cryolite and subjected to electrolysis to extract aluminium.
Why Cryolite is added to alumina?
Alumina is mixed with Cryolite and subjected to electrolysis to extract aluminium.
Which are the ions present in the alumina?
Alumina is mixed with Cryolite and subjected to electrolysis to extract aluminium.
Write the equation of the reduction reaction taking place at negative electrode.
Find relation and fill in the blank:
Bauxite: Leaching
Tinstone: _____.
Which one of the following metals is refined by liquation?
(Zinc, iron, copper,Tin)
Which property of metal is made use of here?
Some important chemical equations of reactions taking place in a blast furnace are given below.
What are the substances fed into the blast furnace along with the ore of iron?
Some important chemical equations of reactions taking place in a blast furnace are given below.
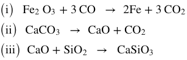
Which compound acts as a reducing agent here.
