EASY
Earn 100
Conjugate acid of is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Ionic Equilibrium
MEDIUM
HARD
MEDIUM
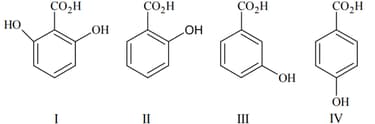
The increasing basicity order of the following compounds is:
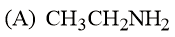
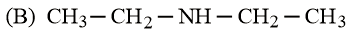
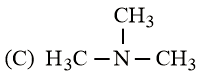
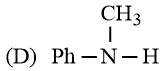
MEDIUM
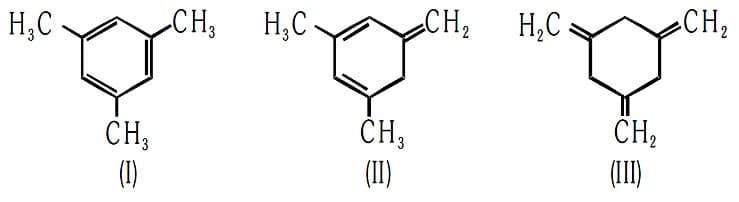
The enthalpy of hydrogenation of these compounds will be in the order as:
EASY
Bond length of ethane ethene ethyne and benzene follows the order:
MEDIUM
MEDIUM
EASY
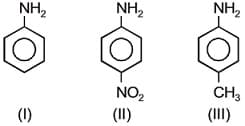
EASY
(i)
(ii)
(iii)
(iv)
EASY
Arrange the following amines in the decreasing order of basicity.
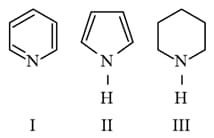
HARD
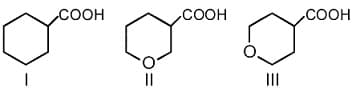
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
EASY

