MEDIUM
Earn 100
Consider a cyclic process for a system. Among the following, the combination of steps that can never lead to this cyclic process is
(a) Adiabatic irreversible; Adiabatic reversible; Adiabatic reversible
(b) Adiabatic irreversible; Adiabatic reversible; : Isothermal reversible
(c) Adiabatic irreversible; Adiabatic reversible; Isothermal irreversible
(d) Isothermal irreversible; Isothermal reversible; Isothermal reversible
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
EASY
MEDIUM
EASY
HARD
EASY
HARD
(: pressure, : volume, : temperature, : enthalpy, : entropy)
EASY
MEDIUM
EASY
Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy
EASY
EASY
Observe the following properties:
Volume, enthalpy, density, temperature, heat capacity, pressure, internal energy.
The number of extensive properties in the above list is
EASY
Reversible process
MEDIUM
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
EASY
(Latent heat of ice is and )
HARD
One mole of an ideal monoatomic gas undergoes two reversible processes and as shown in the given figure:
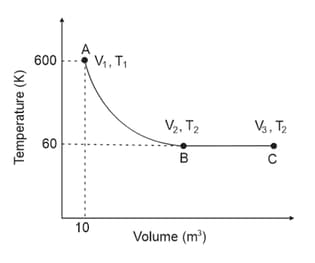
is an adiabatic process. If the total heat absorbed in the entire process and is , the value of is
[Use molar heat capacity of the gas at constant pressure, ]
EASY
EASY
EASY
i)
ii)
iii)
iv)

