HARD
JEE Advanced
IMPORTANT
Earn 100
Consider an ionic solid , with structure. Construct a new structure , whose unit cell is constructed from the unit cell of , following the sequential instructions given below. Neglect the charge balance.
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
50% studentsanswered this correctly

Important Questions on Solid State
HARD
JEE Advanced
IMPORTANT
If the unit cell of a mineral has cubic close packed (ccp) array of oxygen atoms with m fraction of octahedral holes occupied by aluminium ions and n fraction of tetrahedral holes occupied by magnesium ions, m and n, respectively, are
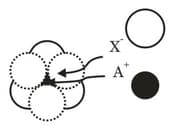
MEDIUM
JEE Advanced
IMPORTANT
The arrangement of X- ions around A+ ion in solid AX is given in the figure (not drawn to scale). If the radius of X- is 250 pm, the radius of A+ is