EASY
Chemistry
IMPORTANT
Earn 100
Consider the addition of and in water as given in (a) and (b)
(a) of in of pure water of
(b) $3.9 \mathrm{mg}$ of $\mathrm{CaF}_{2}$ in $100 \mathrm{~mL}$ of pure water, of . Choose the correct statement(s) from amongst the following.
(a) will completely dissolve
(b) solution will have saturation.
(c) will not completely dissolve
(d) solution will have saturation.
100% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
Consider the equilibria:
1.
2.
(Haemoglobin in blood)
Identify the correct statement(s):
EASY
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
The following represent the variation of with acid strength for aqueous solutions, Marked as and .
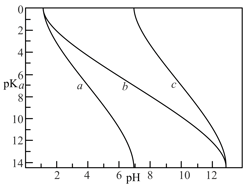
Select the correct matchings:
HARD
Chemistry
IMPORTANT
At , the progress of the reaction:
in a vessel is presented in the following figure:
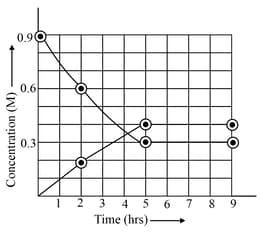
Identify the correct statement(s):
HARD
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
Determine the concentration of solution, one litre of which can dissolve of and of are and respectively.
