MEDIUM
Earn 100
Consider the cell: . The cell potential:
(a)increases on increasing concentration of ions
(b)decreases or decreasing concentration of ions
(c)is independent of concentration of ions
(d)is independent of amounts of and
50% studentsanswered this correctly
Important Questions on Electrochemistry
MEDIUM
Calculate the standard cell potential (in V) of the cell in which the following reaction takes place:
Given that
EASY
Among the following, the strongest reducing agent is:
HARD
The for will be :
EASY
Given the standard half-cell potentials of the following as
Then the standard e.m.f. of the cell with the reaction is
MEDIUM
Some materials are given below:
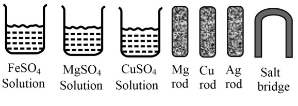
Write the chemical equation fo the reaction taking place at the cathode.
MEDIUM
MEDIUM
Based on these data, which of the following statements is correct?
EASY
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
In which metal container, the aqueous solution of can be stored?
MEDIUM
What will be the oxidation potential for the following hydrogen half cell at bar pressure and temperature?
MEDIUM
MEDIUM
Some materials are given below:
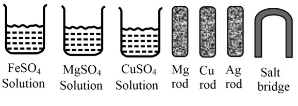
Which is the anode of this cell?
HARD

