EASY
Earn 100
Consider the chemical equilibrium inside a vessel fitted with a movable piston at temperature . The correct plot corresponding to volume change (assuming ideal gas behavior) is
(a)
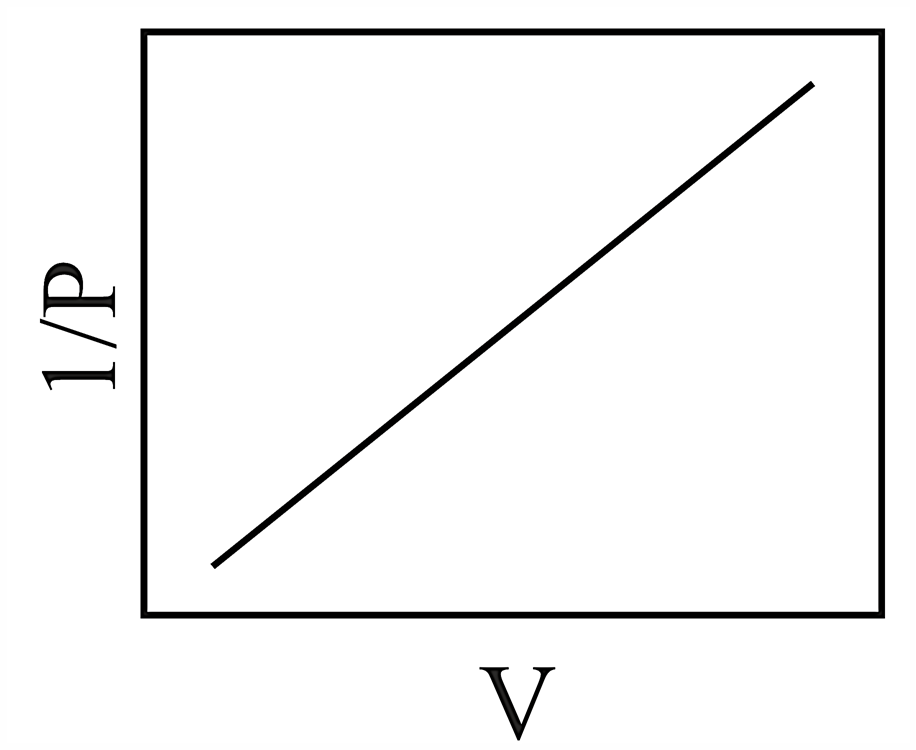
(b)
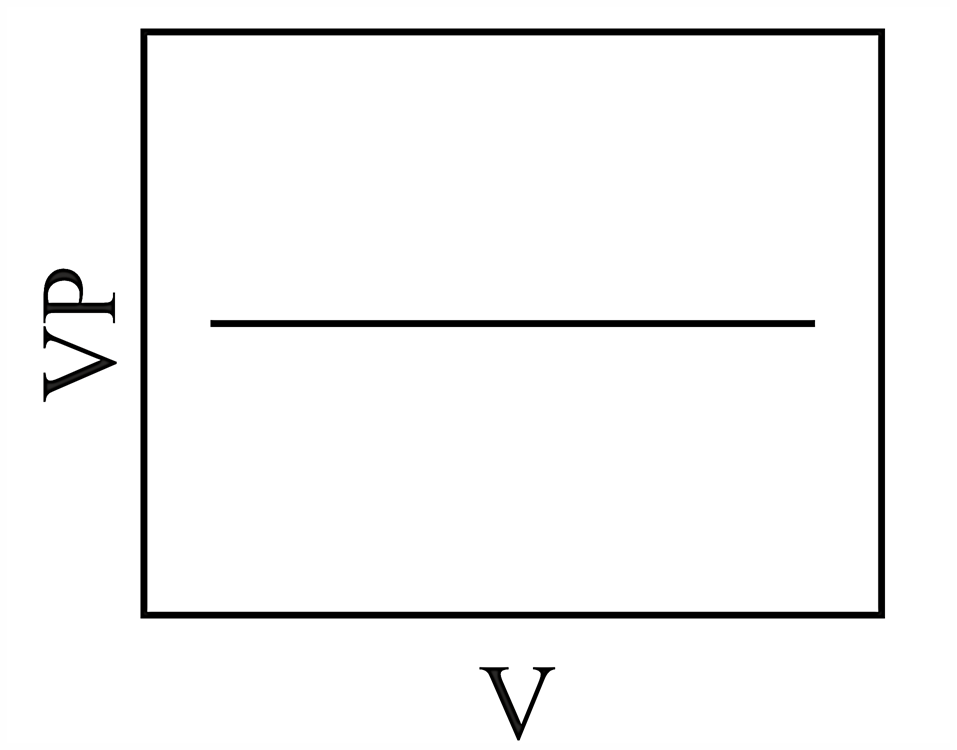
(c)
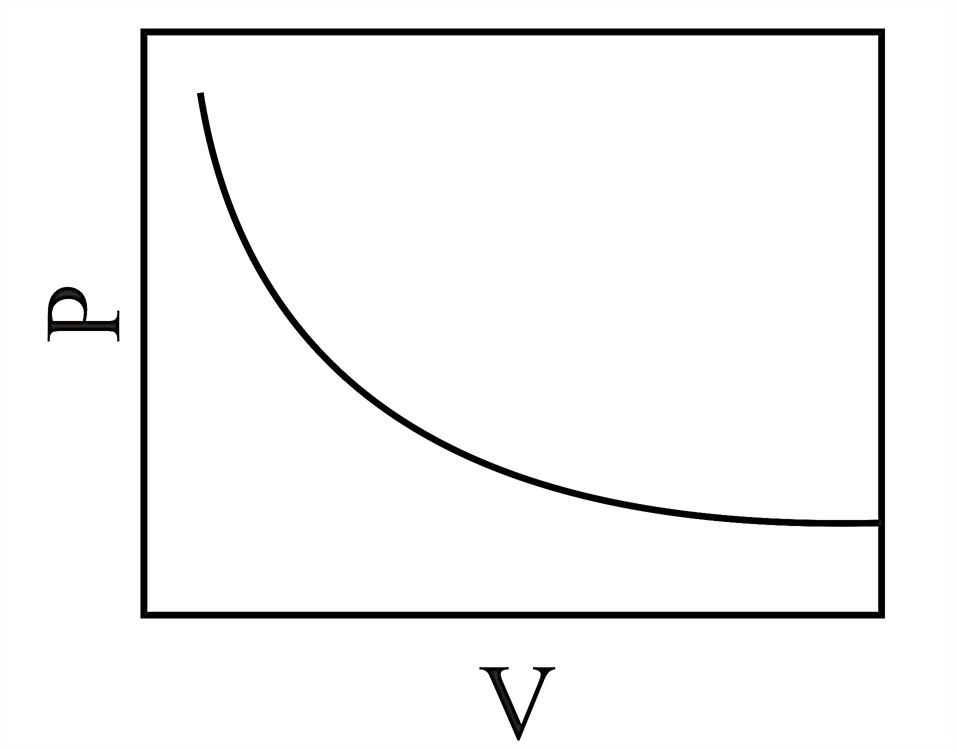
(d)
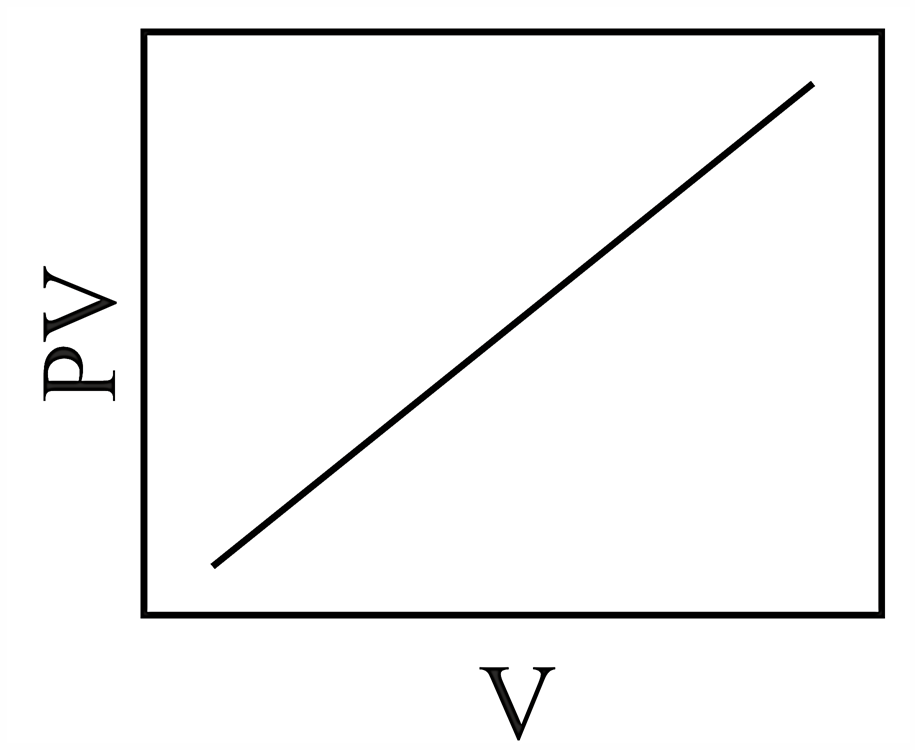
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
HARD
Aluminium reacts with sulfuric acid to form aluminium sulfate and hydrogen. What is the volume of hydrogen gas in liters produced at and atm pressure, when of aluminium and of sulfuric acid are combined for the reaction? (Use molar mass of aluminium as , )
EASY
The value of 1 mole of any pure ideal gas at standard temperature and pressure is always equal to
HARD
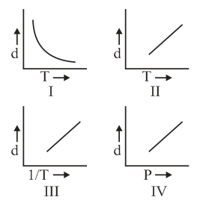
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
EASY
The pressure exerted by a mixture of of methane and of contained in a flask at is
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
MEDIUM
One moles each of four ideal gases are kept as follows.
I. of gas at pressure
II. of gas at pressure
III. of gas at pressure
IV. of gas at pressure
Which of the above gases is kept at highest temperature.
MEDIUM
What is the density of water vapour at boiling point of water?
MEDIUM
A closed container has avogadro number of gaseous molecules at and pressure. The pressure of the same gas at in is
EASY
At , the density of a certain gaseous molecule at bar is double to that of dinitrogen at bar. The molar mass of the gaseous molecule is
MEDIUM
The density of acetic acid vapour at and is . The number of acetic acid molecules in the cluster that is formed in the gas phase is closest to
MEDIUM
grams of a gas at and pressure occupies a volume of . The gas can be
MEDIUM
Assuming ideal gas behavior, the ratio of density of ammonia to that of hydrogen chloride at same temperature and pressure is: (Atomic weight of Cl is 35.5 u)
EASY
One mole of an ideal gas occupies at . What is the pressure of the gas?
MEDIUM
The density of a certain gas at and torr is , then density of the gas at is
EASY
An ideal gas is placed in a tank at . The pressure is initially . One fourth of the gas is then released from the tank and thermal equilibrium is established. What will be the pressure if the temperature is ?
EASY
A container of volume can with stand a maximum pressure of atm at before exploding. The maximum amount of nitrogen (in ) that can be safely put in this container at this temperature is closest to
EASY
At temperature and pressure, the density of a gas is . The gas is
HARD
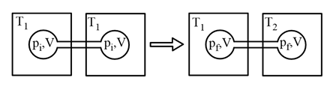
Two closed bulbs of equal volume containing an ideal gas initially at pressure and temperature are connected through a narrow tube of negligible volume, as shown in the figure below. The temperature of one of the bulbs is then raised to The final pressure is:
EASY
10 moles of a mixture of hydrogen and oxygen gases at a pressure of 1 atm at a constant volume and temperature, react to form 3.6 g of liquid water. The pressure of the resulting mixture will be closest to:
MEDIUM
An open vessel at is heated until two fifth of the air (assumed as an ideal gas) in it has escaped from the vessel. Assuming that the volume of the vessel remains constant, the temperature to which the vessel has been heated is:

