EASY
NEET
IMPORTANT
Earn 100
Consider the following
The correct relation between is
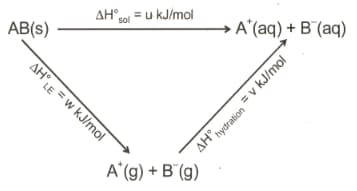
The correct relation between is

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
(a)
(b)
(c)
(d)
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
