HARD
JEE Main/Advance
IMPORTANT
Earn 100
Consider the following Galvanic cell as shown in figure. By what will value the cell voltage change when concentration of ions in anodic and cathodic compartments are both increased by factor of at .
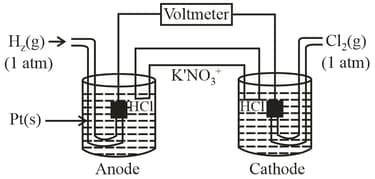

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
For the cell, .
Then calculate approximate value of
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
