HARD
12th ICSE
IMPORTANT
Earn 100
Consider the following cell reaction: and and . What is the cell potential at ?

Important Questions on Electrochemistry
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
One half cell in a voltaic cell is constructed from a silver wire dipped in silver nitrate solution of unknown concentration. Its other half cell consists of a zinc electrode dipping in solution of . A voltage of is measured for this cell. Use this information to calculate the concentration of silver nitrate solution used.
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
The molar conductivity of solutions, at different concentration at , is plotted as shown in the figure given below:
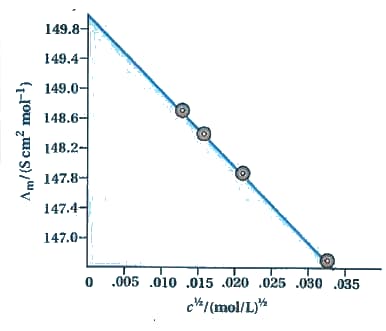
Determine the value of and for .
HARD
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
, atomic mass of ]
