HARD
Earn 100
Consider the following electrochemical cell:
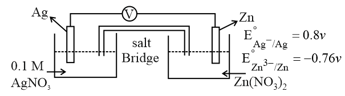
How is the cell potential affected if KI is added to half cell ?

How is the cell potential affected if KI is added to half cell ?
(a)EMF of the cell decreases
(b)Standard EMF of the cell decreases
(c)EMF of the cell increases
(d)Standard EMF of the cell increases
50% studentsanswered this correctly
Important Questions on Electrochemistry
MEDIUM
Based on the data given above, strongest oxidising agent will be:
MEDIUM
when
Given:
The value of is -
HARD
the emf of this Daniel cell is . When the concentration is changed to and that of changed to the emf changes to . From the following, which one is the relationship between and ? (Given, )
HARD
(Given, at )
HARD
EASY
MEDIUM
The emf of the cell is found to be at . The standard potential of half-reaction at will be:
(Given: at )
HARD
Oxidizing power of the species will increase in the order:
MEDIUM
MEDIUM
Given that,
MEDIUM
HARD
EASY
HARD
MEDIUM
Given that,
The strongest oxidizing agent is
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
The reducing power of the metals increases in the order:

