MEDIUM
Earn 100
Consider the following equation for an electrochemical cell reaction. Which of the following changes in condition will increase the cell voltage?
(I) Dissolve concentrated in the cell solution
(II) Increase the pressure of
(III) Increase the amount of
(a)III
(b)I and II
(c)II and III
(d)II
50% studentsanswered this correctly
Important Questions on Electrochemistry
MEDIUM
MEDIUM
HARD
EASY
EASY
MEDIUM
HARD
MEDIUM
HARD
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
EASY
,
MEDIUM
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
EASY
At , the of the galvanic cell mentioned below is
MEDIUM
EASY
MEDIUM
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
MEDIUM
MEDIUM
MEDIUM
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
EASY
EASY
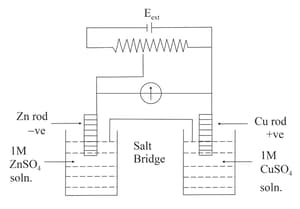
Identify the incorrect statement from the options below for the above cell:

