HARD
Earn 100
Consider the following gaseous reaction where initial pressure of is
After time the pressure of system increased to Calculate the value of (in seconds).
(Given: rate constant of reaction, )
(a)600
(b)300
(c)50
(d)5
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
For an elementary chemical reaction, , the expression for is:
MEDIUM
In the following reaction;
‘A’ and ‘B’ respectively can be:
MEDIUM
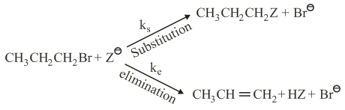
where,
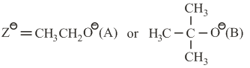
and , are respectively, the rate constants for substitution and elimination, and , the correct option is ________
EASY
Consider the following reactions
The order of the above reactions are respectively. The following graph is obtained when log[rate] vs.log[conc.] are plotted:
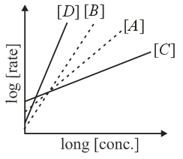
Among the following, the correct sequence for the order of the reactions is :
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
HARD
The results given in the below table were obtained during kinetic studies of the following reaction:
| Experiment | Initial rate/ | ||
| I | |||
| II | |||
| III | |||
| IV | X | ||
| V | Y |
X and Y in the given table are respectively :
EASY
EASY
EASY
If the concentration of is increased from , keeping the value of at , the rate constant will be:
HARD
...(i)
...(ii)
The closest rate constant for the overall reaction
is:
EASY
Which of the following expression is correct for the rate of reaction given below ?
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
The initial concentration of is and it is after 30 minutes. The rate of formation of is:
MEDIUM
(i)
(ii)
(iii)
The overall order of the reaction will be
EASY

