Consider the following process for the conversion of to .
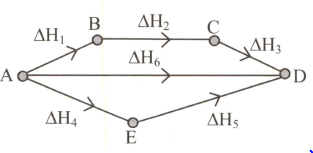
How many of the following represent the correct expressions, according to the Hess's law?

Important Questions on Chemical Thermodynamics and Energetics
(Nearest integer)
Given : .
,
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
At , the enthalpy of the following processes are given:
What would be the value of X for the following reaction? (Nearest integer)
Solid fuel used in rocket is a mixture of and (in ratio ). The heat evolved per gram of the mixture is _____ (Nearest integer)
Given
Molar mass of and are and respectively
Based on the above thermochemical equations, find out which one of the following algebraic relationships is correct?
Consider the following data
Heat of combustion of
Heat of combustion of
Heat of combustion of
The heat of formation of is _____ (Nearest integer).
(Specific heat of water liquid and water vapour are and ; heat of liquid fusion and vaporization of water are and , respectively). ( )
Calculate for formation
Given
(A)
(B)
The for the reaction is
The Born-Haber cycle for is evaluated with the following data:
The magnitude of lattice enthalpy of in is (Nearest integer)
The enthalpy change for the following reaction is

