MEDIUM
JEE Advanced
IMPORTANT
Earn 100
Consider the following reactions, which are carried out at the same temperature.
Which of the following statement is correct about these reactions?
(a)Both the reactions take place at the same rate
(b)The second reaction takes place faster than first reaction
(c)The first reaction takes place faster than second reaction
(d)Both the reactions take place by mechanism.
100% studentsanswered this correctly

Important Questions on Alcohols, Phenols and Ethers
MEDIUM
JEE Advanced
IMPORTANT
In the above reaction, the leaving group is:
HARD
JEE Advanced
IMPORTANT
Which of the following does not represent the correct product?
MEDIUM
JEE Advanced
IMPORTANT
What are the products of the following reaction?
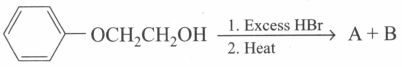
HARD
JEE Advanced
IMPORTANT
Which one of the following will be the most reactive for an reaction?
HARD
JEE Advanced
IMPORTANT
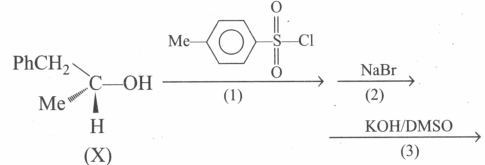
In the following reaction, product is:
MEDIUM
JEE Advanced
IMPORTANT
Which of the following reaction is incorrect?
HARD
JEE Advanced
IMPORTANT
reaction undergoes through a carbocation intermediate as follows:
The correct statements are,
(I) The decreasing order of rate of reaction is .
(II) The decreasing order of ionization energy is .
(III) The decreasing order of energy of activation is .
HARD
JEE Advanced
IMPORTANT
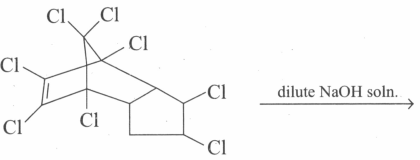
The insecticide chlordane is warmed with dilute solution for some time. The expected product would be: