HARD
Earn 100
Consider the following statement regarding Maxwell's distribution of velocities. The correct statement (s) is / are :
(a)As temperature increases, the peak (maxima) of a curve is shifted towards right side.
(b)The area under the curve at all the temperatures is the same because it represents the number of gaseous molecules.
(c)The fractions of molecules have different velocities are different at a given temperature.
(d)As temperature increases, the most probable velocity of molecules increases but fraction of molecules of maximum velocity decreases.
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
EASY
Two vessels separately contain two ideal gases and at the same temperature, the pressure of being twice that of . Under such conditions, the density of is found to be times the density of . The ratio of molecular weights of and is
MEDIUM
What is the kinetic energy (in ) of the nitrogen molecule at ?
EASY
Two flasks and have equal volumes. is maintained at and at . Equal masses of and are taken in flasks and respectively. Find the ratio of total of gases in flask to that of .
EASY
A molecule of is two times heavier than a molecule. At the average kinetic energy of molecule is
MEDIUM
Which of the following is not an assumption of the kinetic theory of gases?
EASY
What is the average kinetic energy of molecules of an ideal gas leaking freely through an orifice of a container which has molecules at pressure in volume ?
MEDIUM
Among the following, the plot that shows the correct marking of most probable velocity average velocity and root mean square velocity is :
EASY
The ratio of root-mean-square velocity of hydrogen at to that of nitrogen at is closest to
EASY
Number of molecules in a volume of of a perfect monoatomic gas at some temperature and at a pressure of of mercury is close to? (Given, mean kinetic energy of a molecule (at T) is density of mercury )
EASY
Predict the correct order of rate of diffusion of the following molecules.
MEDIUM
Select from the box, the correct gas law related to the given situation.
| Boyle's law, Charles law, Avagadro's law |
An inflated balloon kept at sunlight bursts after some time.
MEDIUM
Points and in the following plot respectively correspond to ( most probable velocity)
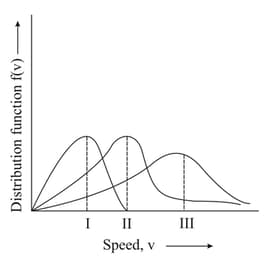
MEDIUM
The speed of the gas molecules contained in a closed vessel at fixed temperature and pressure is the same.
MEDIUM
The order of kinetic diameter of and is:
EASY
For gaseous state, if most probable speed is denoted by , average speed by and root mean square speed by , then for many molecules, what is the ratios of these speeds?
MEDIUM
The temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is : (Atomic masses : He = 4 u, O = 16 u)
EASY
At room temperature, the average speed of Helium is higher than that of Oxygen by a factor of:
MEDIUM
The kinetic energy in of mole of at is:
MEDIUM
Identify the correct labels of and in the following graph from the options given below:
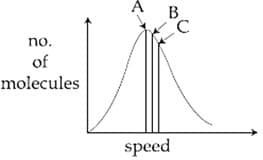
Root mean square speed most proable speed Average speed
MEDIUM
Which of the following statement(s) (are) correct regarding the root-mean-square speed and average translational kinetic energy of a molecule in a gas at equilibrium?

