Consider the following statements
Phenyl diazonium forms azodye with
aniline
phenol
N, N-dimethylaniline
anisole (methoxybenzene)
Important Questions on Organic Compounds Containing Nitrogen
Statement I:
Primary aliphatic amines react with to give unstable diazonium salts.
Statement II :
Primary aromatic amines react with to form diazonium salts which are stable even above . In the light of the above statements, choose the most appropriate answer from the options given below
What is the major product "P" of the following reaction ?
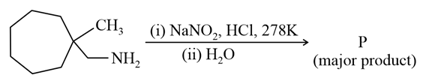
and in the following reactions are:
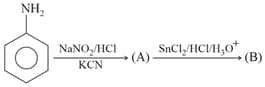
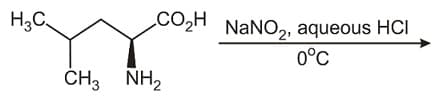
In the reaction
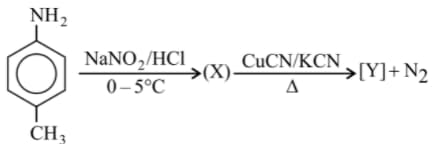
the product is
How will you bring about the following transformations?
Aniline to Benzene diazonium chloride
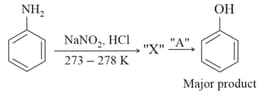
In the above chemical reaction, intermediate "" and reagent/condition "" are :
The correct sequence of correct reagents for the following transformation is :-
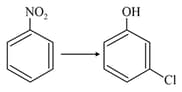
In the following reaction sequence, is :
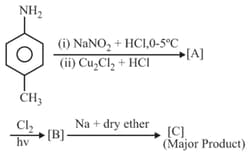
Give the structures of A and B in the following sequence of reactions:
Explain the following :
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
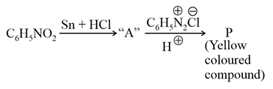
Consider the above reaction, the Product "P" is :
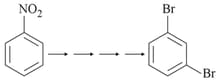
Three isomers A, B and C (mol. formula ) give the following results:
and
R has lower boiling point than S
alkali-insoluble product
and respectively are:
