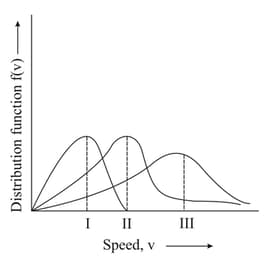
Consider the following statements:
I. Chemical properties of a substance do not change with the change of its physical state.
II. Gases have maximum thermal energy and minimum force of attraction out of three states i.e., Solid, Liquid and Gas.
III. Intermolecular forces are attractive as well as repulsive in nature.
The correct statement(s) is/are
Important Questions on States of Matter: Gaseous and Liquid States
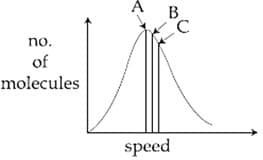
Root mean square speed most proable speed Average speed
Select from the box, the correct gas law related to the given situation.
| Boyle's law, Charles law, Avagadro's law |
An inflated balloon kept at sunlight bursts after some time.
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as , where is the volume of the gas. The value of is:
According to kinetic molecular theory of gases, which of the following statements are correct?
a) The actual volume of the molecules is negligible in comparison to the empty space between them.
b) Collisions of gas molecules are inelastic.
c) At any particular time, different particles in the gas have the same speed and same kinetic energies.
d) Pressure is exerted by the gas as a result of collision of the particles with the walls of the container.