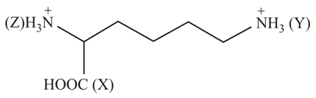
MEDIUM
JEE Main
IMPORTANT
Earn 100
Consider the following three halides:
()
()
()
Arrange the bond length of these compounds in the decreasing order.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main
IMPORTANT
The order of acidity of compounds is
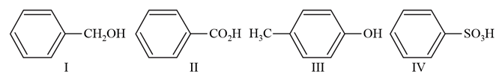
MEDIUM
JEE Main
IMPORTANT
Among the following substituted pyridines, the most basic compound is:
MEDIUM
JEE Main
IMPORTANT
The correct order of acidity of the following compounds is:
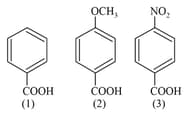
MEDIUM
JEE Main
IMPORTANT
Among the following sets, the most stable ionic species are:
MEDIUM
JEE Main
IMPORTANT
The correct order of the acid strength of the following compounds:
(A) Phenol
(B) -Cresol
(C) -Nitrophenol
(D) -Nitrophenol is :
MEDIUM
JEE Main
IMPORTANT
Among the following, the strongest acid is:
MEDIUM
JEE Main
IMPORTANT
Among the following the correct order of basicity is:
MEDIUM
JEE Main
IMPORTANT
The correct order of acidity of the three groups (marked ) in the given species is: