MEDIUM
JEE Main
IMPORTANT
Earn 100
Consider the given plot of enthalpy of the following reaction between and
Identify the incorrect statement.
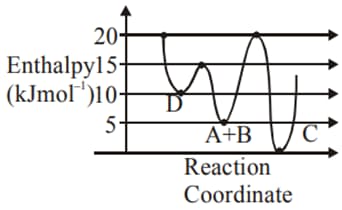
(a)Formation of and from has highest enthalpy of activation.
(b) is kinetically stable product.
(c) is the thermodynamically stable product.
(d)Activation enthalpy to form is less than that to form
25% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
MEDIUM
JEE Main
IMPORTANT
The rate of a reaction quadruples when the temperature changes from to . The activation energy of this reaction is:
(Assume Activation energy and pre-exponential factor are independent of temperature; )
HARD
JEE Main
IMPORTANT
The reaction of ozone with oxygen atoms in the presence of chlorine atoms can occur by a two step process shown below:
...(i)
...(ii)
The closest rate constant for the overall reaction
is:
HARD
JEE Main
IMPORTANT
The half-life period of a first-order reaction is . The amount of substance left after one hour will be
MEDIUM
JEE Main
IMPORTANT
Consider the following plots of rate constant versus for four different reactions. Which of the following orders is correct for the activation energies of these reactions?
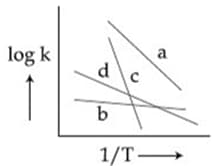
MEDIUM
JEE Main
IMPORTANT
The rate of a certain biochemical reaction at physiological temperature occurs times faster with enzyme than without. The change in the activation energy upon adding enzyme is:
HARD
JEE Main
IMPORTANT
For a reaction scheme if the net rate of formation of B is set to be zero then the concentration of B is given by:
MEDIUM
JEE Main
IMPORTANT
For the reaction , the values of initial rate at different reactant concentrations are given in the table below. The rate law for the reactions is:
| 0.05 | 0.05 | 0.045 |
| 0.10 | 0.05 | 0.090 |
| 0.20 | 0.10 | 0.72 |
