HARD
Chemistry
IMPORTANT
Earn 100
Consider the given plots for four gases at . If the slope of line is , what is the relationship between Vander Waal's constants '' and ''? (Ignore the higher terms of Virial equation)
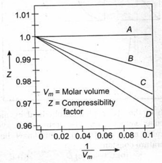

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
One mole of is taken in litre empty container fitted with a movable piston at If it is heated to at constant pressure then match the change (List) in parameters (List ) of gas as compared to initial state select the correct code.
| List (Parameter) | List (Change) | ||
| (number of collision made by a molecule per unit time) | |||
| (collision frequency) | |||
| (mean free path) | |||
| (root mean square speed) | |||
MEDIUM
Chemistry
IMPORTANT
us curves at constant and for an ideal gas are shown in figure. Which is correct :
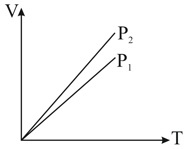
MEDIUM
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
