EASY
Earn 100
Consider the molecule:
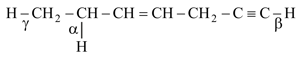
The order of bond energy is:
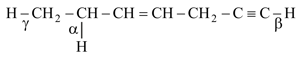
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
Which one of the following esters gets hydrolyzed most easily under alkaline conditions?
MEDIUM
The correct statement regarding the basicity of aryl amines is:
MEDIUM
The increasing basicity order of the following compounds is:
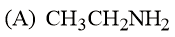
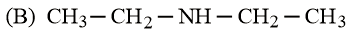
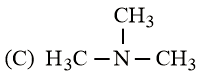
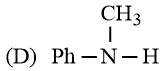
HARD
The correct order of strengths of carboxylic acids is
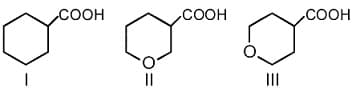
EASY
Bond length of ethane ethene ethyne and benzene follows the order:
MEDIUM
Given,
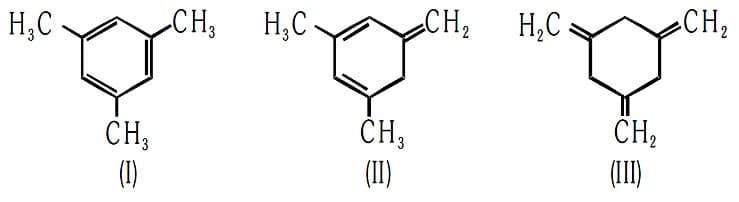
The enthalpy of hydrogenation of these compounds will be in the order as:
MEDIUM
The correct order for acid strength of compounds and is as follows:
EASY
Among the following oxoacids, the correct decreasing order of acid strength is :
EASY
The order of stability of the following alkenes with the first being the most stable and last being the least stable is
(i)
(ii)
(iii)
(iv)
MEDIUM
Correct statements for the given reaction are:
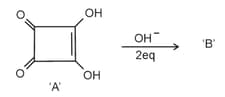
A. Compound ‘’ is aromatic
B. The completion of above reaction is very slow
C. ‘’ shows tautomerism
D. The bond lengths of in compound are found to be same
Choose the correct answer from the options given below.
EASY
In case of substituted aniline the group which decreases the basic strength is
MEDIUM
Which of the following has the shortest bond?
MEDIUM
The compound that does NOT liberate CO2, on treatment with aqueous sodium bicarbonate solution, is
MEDIUM
The correct order of basicity is
EASY
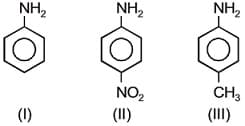
The correct increasing order of the basic strength for the following compounds is:
HARD
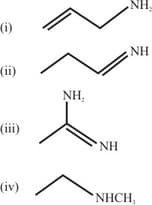
The increasing order of basicity of the following compounds is :
MEDIUM
Considering the basic strength of amines in an aqueous solution, which one has the smallest value?
HARD
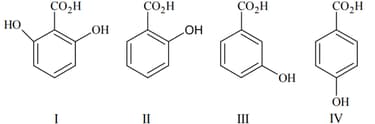
The correct order of acidity for the following compounds is :
MEDIUM
Among the following compounds, which one has the shortest bond?
EASY
Which of the following molecules form a Zwitter ion?

