HARD
JEE Main
IMPORTANT
Earn 100
Consider the reversible isothermal expansion of an ideal gas in a closed system at two different temperatures and . The correct graphical depiction of the dependence of work done vs the final volume is:
(a)
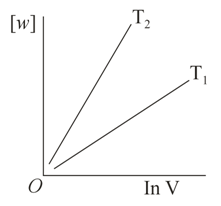
(b)
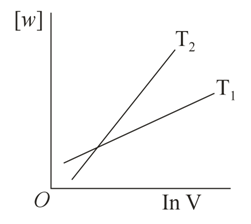
(c)
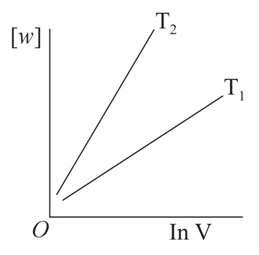
(d)
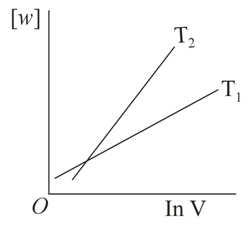
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
During compression of a spring the work done is and escaped to the surroundings as heat. The change in internal energy, is:
EASY
JEE Main
IMPORTANT
Among the following, the set of parameters that represents path functions, is:
i)
ii)
iii)
iv)
EASY
JEE Main
IMPORTANT
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
EASY
JEE Main
IMPORTANT
A gas undergoes change from state to state . In this process, the heat absorbed and work done by the gas is , respectively. Now gas is brought back to by another process during which of heat is evolved. In this reverse process of .
MEDIUM
JEE Main
IMPORTANT
For the reaction,
and are, respectively, and at 298 K. The equilibrium constant for the reaction at 298 k is:
EASY
JEE Main
IMPORTANT
A reaction at 1 bar is non-spontaneous at low temperature but becomes spontaneous at high temperature. Identify the correct statement about the reaction among the following:
MEDIUM
JEE Main
IMPORTANT
The standard enthalpy of formation of is . If bond enthalpy of is and that of is , the average bond enthalpy of bond in is :
MEDIUM
JEE Main
IMPORTANT
At constant volume, of an ideal gas when heated from to changes its internal energy by The molar heat capacity at constant volume is ________
