EASY
JEE Main/Advance
IMPORTANT
Earn 100
Consider two liquids having pure vapour pressures forming an ideal solution. The plot of (where and are the mole fraction of liquid in liquid and vapour phase respectively) is linear with slope and intercepts respectively:
(a) and
(b) and
(c) and
(d) and
43.1% studentsanswered this correctly

Important Questions on Solutions
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
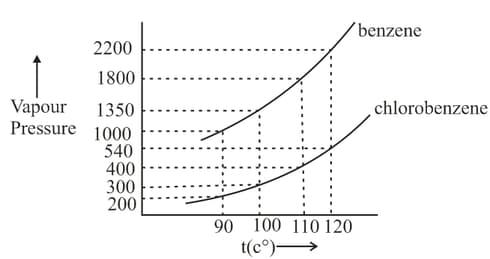
Assuming the formation of an ideal solution, determine the boiling point of a mixture containing benzene (molar mass ) and chlorobenzene (molar mass ) using the following against an external pressure of Torr.
MEDIUM
JEE Main/Advance
IMPORTANT
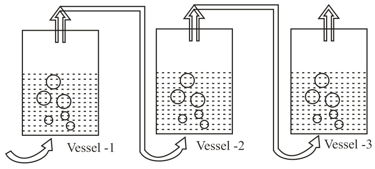
Dry air is slowly passed through three solutions of different concentrations, and ; each containing (non volatile) as solute and water as solvent, as shown in the Fig. If the vessel gains weight and the vessel loses weight, then
MEDIUM
JEE Main/Advance
IMPORTANT
