EASY
Earn 100
Consider two reactions
It is given that all the energy released from the conversion of to is used in the oxidation of to . Under standard conditions, on conversion of 1 mole of moles of are oxidised. The value of is (use )
20% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
MEDIUM
EASY
,
HARD
The reaction occurs in which of the given galvanic cell?
EASY
At , the of the galvanic cell mentioned below is
EASY
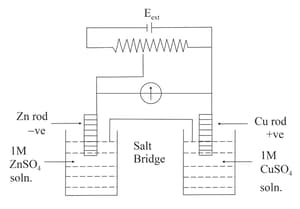
Identify the incorrect statement from the options below for the above cell:
HARD
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
MEDIUM
EASY
HARD
Calculate the change in the cell potential after the passage of 9.65 A of current for 1 h.
HARD
The following electrochemical cell has been set up.
If an ammeter is connected between the two platinum electrodes, predict the direction of flow of current.
MEDIUM
EASY
HARD
EASY
EASY
EASY
EASY
MEDIUM
HARD
Statement II : The function of salt bridge is to remove liquid-liquid junction potential because the salt used has same speed of cations and anions.

