HARD
Earn 100
Consider two reactions having same Arrhenius factor A, but different energies of activation.
(i)
(ii)
Both are at temperature
If temperature in both reaction is increased slightly in such a way that change in temperature in both case is same then, choose the correct option.
(a)The second reaction is faster
(b)The second reaction is more sensitive towards temperature variation
(c)If temperature increases, rate of first reaction increase more sharply
(d)All the above are correct
54.55% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
(Assume Activation energy and pre-exponential factor are independent of temperature; )
MEDIUM
MEDIUM
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
The Arrhenius plots of two reactions, I and II are shown graphically-
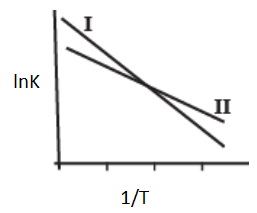
The graph suggests that-
EASY
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY
EASY
MEDIUM
EASY
MEDIUM
Identify the incorrect statement.
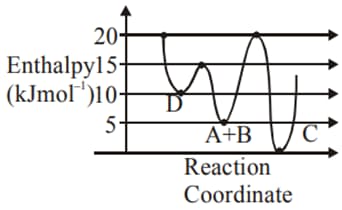
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
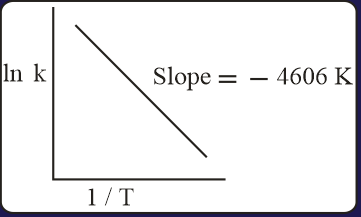
MEDIUM
MEDIUM
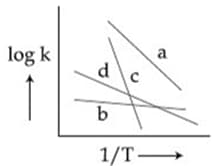
MEDIUM
MEDIUM
MEDIUM
[Gas constant, ]
EASY

