HARD
JEE Main/Advance
IMPORTANT
Earn 100
Consider -axis as internuclear axis. How many of the following will lead to bond formation:
(i) (ii) (iii) (iv) (v) (vi) (vii) (viii)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main/Advance
IMPORTANT
Which of the following Lewis diagram is/are incorrect?
MEDIUM
JEE Main/Advance
IMPORTANT
Which are the exceptions to the Lewis octet rule?
MEDIUM
JEE Main/Advance
IMPORTANT
Which species have same bond order?
MEDIUM
JEE Main/Advance
IMPORTANT
If the given compound is planar. Select the correct statement.
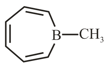
HARD
JEE Main/Advance
IMPORTANT
Among the above compounds, compounds having at least one bond are
Among the above compounds, compounds having at least one bond are
Give the answer as .
EASY
JEE Main/Advance
IMPORTANT
Molecular shapes of and are respectively:
EASY
JEE Main/Advance
IMPORTANT
The number of lone pair(s) of electrons in are:
HARD
JEE Main/Advance
IMPORTANT
The compound (s) with two lone pairs of electrons on the central atom is (are)
