MEDIUM
Earn 100
Considering the reaction of butyl bromide with ion:
.
Assuming no other change, what effect on the rate would result from simultaneously doubling the concentration of both butyl bromide and ion?
(a)It would double the rate
(b)It would increase the rate by six times
(c)It would increase the rate to four times
(d)It would triple the rate
87.5% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
EASY
MEDIUM
EASY
EASY
HARD
EASY
(a) In which test tube does the reaction proceed faster?
(b) Give reason.
(c) Give an instance in daily life, where such condition is made use.
MEDIUM
Which one of the following statements is correct?
HARD
(i) time, (ii) concentration of reactants, (iii) temperature, and (iv) catalyst
MEDIUM
MEDIUM
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)
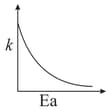
(II)
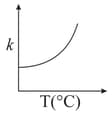
EASY
EASY
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
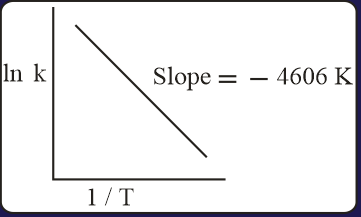
HARD
For an elementary chemical reaction, , the expression for is:
EASY
MEDIUM
MEDIUM

