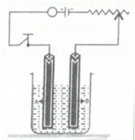
Copper sulphate solution is electrolysed using a platinum anode.
Study the diagram given alongside and answer the following questions:
Give the names of the electrodes A and B.


Important Questions on Electrolysis
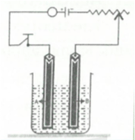
Copper sulphate solution is electrolysed using a platinum anode.
Study the diagram given alongside and answer the following question:
Which electrode is the oxidizing electrode ?
To carry out the so-called "electrolysis of water". Sulphuric acid is added to water.
How does the addition of sulphuric acid produce a conducting solution ?
Electrolysis of acidulated water is an example of _____reaction (Reduction/oxidation/redox/synthesis).
Explain the following:
A solution of cane sugar does not conduct electricity, but a solution of sodium chloride is a good conductor.
Explain the following: Hydrochloric acid is a good conductor of electricity.
Explain the following:
During the electrolysis of an aqueous solution of , hydrogen ion is reduced at the cathode and not the sodium ion though both and ions are present in the solution.
Explain the following: On electrolysis of dilute copper (II) sulphate solution, copper is deposited at the cathode but no hydrogen gas evolves there. Explain why?
When a dilute aqueous solution of sodium chloride is electrolysed between platinum electrodes, hydrogen gas is evolved at the cathode but metallic sodium is not deposited. Why?
