EASY
Earn 100
Correct match for the graph between intensity as frequency for X-rays will be:
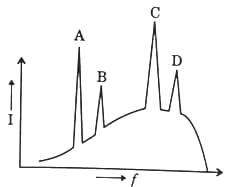

(a)
(b)$A \rightarrow K_{\beta}, B \rightarrow K_{\alpha}, C \rightarrow L_{\alpha}, D \rightarrow L_{\beta}$
(c)$A \rightarrow K_{\alpha}, B \rightarrow K_{\beta}, C \rightarrow L_{\alpha}, D \rightarrow L_{\beta}$
(d)$A \rightarrow L_{\beta}, B \rightarrow L_{\alpha}, C \rightarrow K_{\beta}, D \rightarrow K_{\alpha}$
100% studentsanswered this correctly
Important Questions on Atoms and Nuclei
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
HARD
MEDIUM
MEDIUM

