EASY
JEE Main
IMPORTANT
Earn 100
and are specific heats at constant pressure and constant volume respectively. It is observed that, for hydrogen gas and for nitrogen gas. The correct relation between and is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
An ideal gas has molecules with degrees of freedom. The ratio of specific heats at constant pressure and at constant volume is:
HARD
JEE Main
IMPORTANT
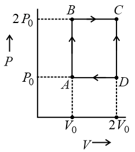
An engine operates by taking moles of an ideal gas through the cycle shown in figure. The thermal efficiency of the engine is:
(Take , where is gas constant)
MEDIUM
JEE Main
IMPORTANT
moles of diatomic gas in a cylinder is at a temperature . Heat is supplied to the cylinder such that the temperature remains constant but moles of the diatomic gas get converted into monoatomic gas. The change in the total kinetic energy of the gas is
EASY
JEE Main
IMPORTANT
The ratio of work done by an ideal monoatomic gas to the heat supplied to it in an isobaric process is
MEDIUM
JEE Main
IMPORTANT
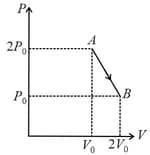
moles of an ideal gas undergoes a process as shown in the figure. The maximum temperature of the gas during the process will be:
HARD
JEE Main
IMPORTANT
An ideal gas undergoes a quasi-static, reversible process in which its molar heat capacity C remains constant. If during this process the relation of pressure P and volume V is given by constant, then n is given by (Here and are molar specific heat at constant pressure and constant volume, respectively) :
MEDIUM
JEE Main
IMPORTANT
A Carnot freezer takes heat from water at inside it and rejects it to the room at a temperature of The latent heat of ice is If 5kg of water at is converted into ice at by the freezer, then the energy consumed by the freezer is close to :
MEDIUM
JEE Main
IMPORTANT
An ideal gas goes through a reversible cycle has the V - T diagram shown below. Process are adiabatic.
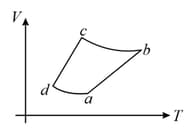
The corresponding P - V diagram for the process is (all figures are schematic and not drawn to scale) :
