MEDIUM
11th CBSE
IMPORTANT
Earn 100
has structure as shown:
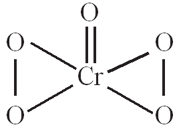
The oxidation number of chromium in the above compound is

(a)
(b)
(c)
(d)
42.86% studentsanswered this correctly

Important Questions on Redox Reactions
MEDIUM
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
are:
EASY
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
(i)
(ii)
The for the reaction (In Volts), is
EASY
11th CBSE
IMPORTANT
The compound which shows superconductivity, has copper in oxidation state _____ . Assume that the rare earth element ytterbium is in the usual oxidation state.
EASY
11th CBSE
IMPORTANT
