MEDIUM
JEE Advanced
IMPORTANT
Earn 100
Crystal radius of three ions have and , respectively. If all the three ions have the same number of protons, select the correct statement.
(a)All the three ions are isoelectronic
(b)All the three ions have same charge
(c)
All the three ions have different charge but metal is same
(d)All are correct
20% studentsanswered this correctly

Important Questions on Periodic Classification of Elements and General Inorganic Chemistry
MEDIUM
JEE Advanced
IMPORTANT
Which of the following orders of atomic/ionic radius are correct?
MEDIUM
JEE Advanced
IMPORTANT
In which of the following pairs size of element is higher as compare to ?
EASY
JEE Advanced
IMPORTANT
Incorrect order of ionic size of elements:
EASY
JEE Advanced
IMPORTANT
Which of the following represents correct order of radius?
EASY
JEE Advanced
IMPORTANT
Which of the following statements(s) is/are correct?
MEDIUM
JEE Advanced
IMPORTANT
is a crystalline solid. It is a molecular solid in which molecules are held together with van der Waals forces. Given diagram represents two adjacent molecules in solid.
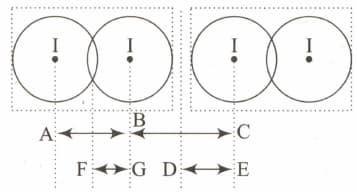
If length and covalent radius of iodine is
Find the Van der Waals radius of atom.
EASY
JEE Advanced
IMPORTANT
is a crystalline solid. It is a molecular solid in which molecules are held together with van der Waals forces. Given diagram represents two adjacent molecules in solid.
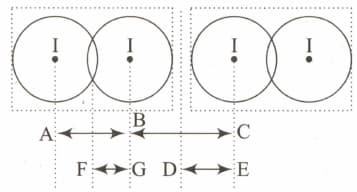
If length and covalent radius of iodine is
Which of the following data is smaller as compared toEASY
JEE Advanced
IMPORTANT
and lengths represent, respectively:
