HARD
Earn 100
Deduce Boyle's law, using the expression for pressure exerted by the gas
Important Questions on Behaviour of Perfect Gases and Kinetic Theory of Gases
HARD
If of gas A contains molecules, how many molecules of gas will be present in of ? The gases and are under the same conditions of temperature and pressure. Name the law on which the problem is based.
EASY
When one litre of an ideal gas at is heated at a constant pressure to a temperature of find its final volume.
HARD
Deduce Boyle’s law using the expression for pressure exerted by the gas.
EASY
How many molecules of will be produced when reacts completely with ?
MEDIUM
Initially a gas of diatomic molecules is contained in a cylinder of volume at a pressure and temperature . Assuming that of the molecules get dissociated causing a change in number of moles. The pressure of the resulting gas at temperature when contained in a volume is given by . The ratio is -
EASY
State whether the below given statement is true or false:
“The volume of gas molecules is taken into consideration in Avogadro’s Law.”
HARD
Calculate the amount of each reactant required to produce of carbon dioxide, when two volumes of carbon monoxide combine with one volume of oxygen to produce two volumes of carbon dioxide.
State the law associated with this question.
EASY
An air bubble of volume rises from the bottom of a lake deep to the surface at a temperature of The atmospheric pressure is the density of water is and There is no difference of the temperature of water at the depth of and on the surface. The volume of air bubble when it reaches the surface will be
EASY
Two gases having same pressure and volume are mixed at a temperature . If the mixture is at a temperature and occupies the same volume then pressure of the mixture would be
MEDIUM
The volume of an enclosure contains a mixture of three gases, of oxygen, of nitrogen and of carbon dioxide at absolute temperature . Consider as universal gas constant. The pressure of the mixture of gases is :
EASY
State Avogadro's law. At a certain fixed temperature and pressure the molar volumes () of the real gases are nearly equal and at STP the limit is . How Avogadro's law can be arrived at from this information obtained from experiments?
HARD
An open glass tube is immersed in mercury in such a way that a length of extends above the mercury level. The open end of the tube is then closed and sealed and the tube is raised vertically up by additional . What will be length of the air column above mercury in the tube now ?
(Atmospheric pressure = of Hg)
MEDIUM
A certain amount of gas of volume at temperature and pressure expands isothermally until its volume gets doubled. Later it expands adiabatically until its volume gets redoubled. The final pressure of the gas will be (Use )
EASY
A container with insulating walls is divided into equal parts by a partition fitted with a valve. One part is filled with an ideal gas at a pressure and temperature , whereas the other part is completely evacuated. If the valve is suddenly opened, the pressure and temperature of the gas will be
EASY
A bicycle tyre is filled with air having pressure of at . The approximate pressure of the air in the tyre when the temperature increases to is
HARD
A container of fixed volume has a mixture of one mole of hydrogen and one mole of helium in equilibrium at temperature . Assuming the gases are ideal, the correct statement(s) is (are)
EASY
The molecules of a given mass of gas have RMS velocity of at and pressure. When the temperature and pressure of the gas are respectively, and , the r.m.s. velocity of its molecules in is:
MEDIUM
A cylindrical container of volume contains one mole of hydrogen and two moles of carbon dioxide. Assume the temperature of the mixture is . The pressure of the mixture of gases is :
[Take gas constant as
EASY
Equal masses of and methane have been taken in a container of volume at temperature in identical conditions. The ratio of the volumes of gases methane would be
EASY
For the diagram given for an ideal gas
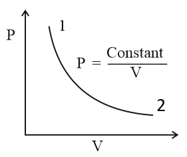
Out of the following which one correctly represents the diagram?

