EASY
Earn 100
Define adsorbate.
Important Questions on Surface Chemistry
MEDIUM
Given:
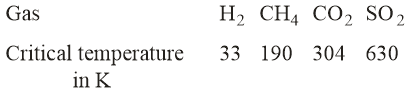
On the basis of data given above, predict which of the following gases shows the least adsorption on a definite amount of charcoal?
MEDIUM
EASY
( is the mass of the gas adsorbed per gram of adsorbent)
HARD
MEDIUM
Define the following term : Adsorbent
MEDIUM
EASY
EASY
MEDIUM
Write any two differences between Absorption and Adsorption.
MEDIUM
EASY
EASY
MEDIUM
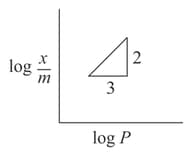
MEDIUM
EASY
Adsorption of the gas increases with:
EASY
Increasing order of boiling points in the following compounds is:
EASY
MEDIUM
of is adsorbed on of platinum powder at and bar pressure. The volume of the gas adsorbed per gram of the adsorbent is
(Given : bar )
MEDIUM
Write three differences between adsorption and absorption.
EASY
of oxygen is adsorbed on of platinum metal. The volume of oxygen adsorbed per gram of the adsorbent at and in is

