EASY
Earn 100
Define buffer solution. What is it mainly used for?
Important Questions on Further Aspects of Equilibria
MEDIUM
Which of the following mixtures will have the lowest pH at
HARD
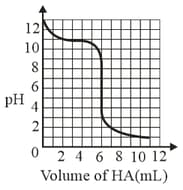
A solution of weak base is titrated with of a strong acid . The variation of of the solution with the volume of added is shown in the figure below. What is the of the base? The neutralisation reaction is given by .
MEDIUM
MEDIUM
[Given:
HARD
The pH of the solution after Expt. 2 is
HARD
HARD
MEDIUM
MEDIUM
HARD
[Given: of acetic acid molar mass of acetic acid , Neglect any changes in volume]
EASY
EASY
MEDIUM
EASY
HARD
EASY
MEDIUM
MEDIUM
MEDIUM
EASY

