MEDIUM
Earn 100
Define positron.
Important Questions on Energy
EASY
EASY
MEDIUM
HARD
MEDIUM
In the decay sequence:
and are the particles/ radiation emitted by the respective isotopes. The correct option(s) is/are:
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
In the radioactive decay process
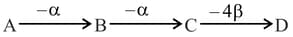
1. A and B are isobars
2. A and D are isotopes
3. C and D are isobars
4. A and C are isotopes
HARD
EASY
The ionisation power is maximum for:
EASY
The explosion of atom bomb is based on the principle of
EASY
HARD
MEDIUM
