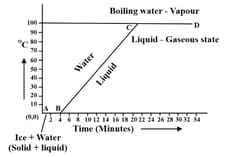
EASY
Earn 100
Define the term specific latent heat of vaporization.
Important Questions on Heat
EASY
MEDIUM
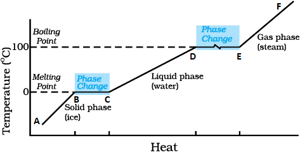
Identify the part representing latent heat of fusion in the temperature vs heat graph for melting of ice.
MEDIUM
MEDIUM
HARD
How much ice at must be dropped into a cup containing of water at in order for the temperature of the water to be reduced to ? The cup itself has a mass of and is made out of aluminium. Assume that no energy is lost to the surroundings.
Specific heat capacity of ice specific latent heat of fusion of ice specific heat capacity of water
MEDIUM
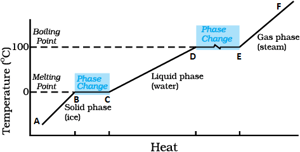
Identify the part representing latent heat of vaporization in the temperature vs heat graph for melting of ice.
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
Explain the following equation to show the meaning of each symbol. Give the unit of each quantity.