MEDIUM
NEET
IMPORTANT
Earn 100
Density of solution of a non-electrolyte is . If is , solution freezes at
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
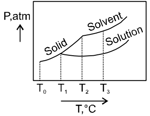
What is the normal freezing point of the solution represented by the phase diagram?
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
