MEDIUM
Earn 100
Derive the formula for the molar specific heat capacity at constant volume of a mixture of two gases.
Important Questions on Molecular Properties of Matter
MEDIUM
Two different metal bodies and of equal mass are heated at a uniform rate under similar conditions. The variation of temperature of the bodies is graphically represented as shown in the figure. The ratio of specific heat capacities is:
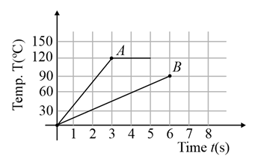
EASY
MEDIUM
MEDIUM
Match the following: (where is gas constant)
| Column I | Column II | ||
| (a) | Molar specific heat of helium gas at constant volume | (i) | |
| (b) | Molar specific heat of oxygen at constant volume | (ii) | |
| (c) | Molar specific heat of carbon dioxide at constant volume | (iii) | |
| (d) | Molar specific heat of hydrogen at constant pressure | (iv) |
HARD
HARD
For a monoatomic ideal gas following the cyclic process shown in the vs plot, identify the incorrect option:

MEDIUM
MEDIUM
HARD
MEDIUM
HARD
mole of gas with is mixed with mole of gas with , then the value of for the resulting mixture is
EASY
EASY
EASY
EASY
A monoatomic gas performs a work of , where is the heat supplied to it. The molar heat capacity of the gas will be _____ during this transformation, where is the gas constant.
EASY
EASY
MEDIUM
EASY
EASY

