EASY
Upper Secondary: IGCSE
IMPORTANT
Earn 100
Describe how dry crystals of zinc chloride can be obtained from a solution of zinc chloride.

Important Questions on Cambridge IGCSE Exam Questions from Paper 2
EASY
Upper Secondary: IGCSE
IMPORTANT
A student electrolysed molten zinc chloride. State the name of the product formed at the anode.
EASY
Upper Secondary: IGCSE
IMPORTANT
A student electrolysed molten zinc chloride. State the name of the product formed at the cathode.
EASY
Upper Secondary: IGCSE
IMPORTANT
When Group elements react with water, hydrogen gas is given off. The diagram shows the reaction of lithium, potassium and sodium with water.
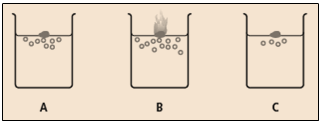
Which one of these three elements A, B or C is lithium?
EASY
Upper Secondary: IGCSE
IMPORTANT
Apart from fizzing, describe two things that you would see when sodium reacts with water.
EASY
Upper Secondary: IGCSE
IMPORTANT
After the sodium had reacted with the water, the solution was tested with red litmus paper. What colour did the litmus paper turn? Give a reason for your answer.
EASY
Upper Secondary: IGCSE
IMPORTANT
Which two of the following statements about sodium are true?
EASY
Upper Secondary: IGCSE
IMPORTANT
Rubidium also reacts with water. How does the speed of reaction of rubidium with water compare with that of potassium with water?
EASY
Upper Secondary: IGCSE
IMPORTANT
Sodium has only one stable isotope whereas potassium has several isotopes. What do you understand by the term isotopes?
