Describe the chemical test for an unsaturated hydrocarbon. Give the result of the test.

Important Questions on Cambridge IGCSE Exam Questions from Paper 2
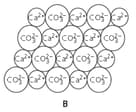
State the chemical name of structure B.
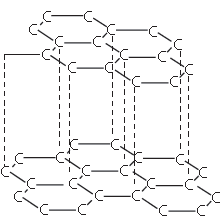
The structure shown below has several uses. Which one of the following is a correct use of the given structure:
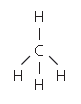
The structures A and E are compounds. What do you understand by the term compound?
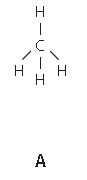
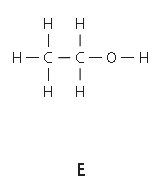
State the type of bonding in given structure.
The table shows observations about the reactivity of various metals with dilute hydrochloric acid.
| metal | observations |
| calcium | many bubbles produced rapidly with much spitting |
| copper | no bubbles formed |
| iron | a few bubbles produced very slowly |
| magnesium | many bubbles produced rapidly with no spitting |
Put the metals in order of increasing reactivity.
The table shows observations about the reactivity of various metals with dilute hydrochloric acid.
| metal | observations |
| calcium | many bubbles produced rapidly with much spitting |
| copper | no bubbles formed |
| iron | a few bubbles produced very slowly |
| magnesium | many bubbles produced rapidly with no spitting |
Zinc is between iron and magnesium in reactivity. Suggest what observations will be made when zinc reacts with dilute hydrochloric acid.
Magnesium is extracted by the electrolysis of molten magnesium chloride.
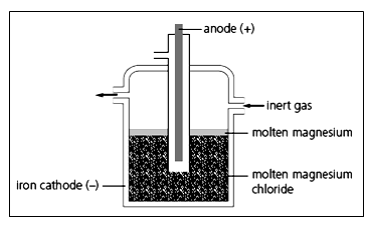
What information in the diagram suggests that magnesium is less dense than molten magnesium chloride?
Magnesium is extracted by the electrolysis of molten magnesium chloride.
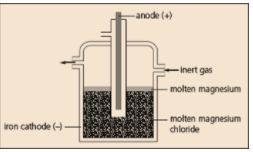
Magnesium is extracted by electrolysis rather than by heating its oxide with carbon. Why?
