MEDIUM
Earn 100
Describe the test of calcium ion with sodium hydroxide.
Important Questions on In the Lab
HARD
then metal M will be
HARD
MEDIUM
Some reactions are given for salt(X).
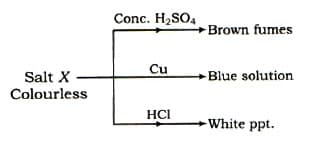
Which of the following salt can be satisfied all the conditions?
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
HARD
The solution will :
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
EASY
