Devise a three-stage synthetic route to convert benzene into benzenediazonium chloride.

Important Questions on Organic Synthesis
A sample of lactic acid, , was extracted from a natural source and found to be optically active. The structure of one optical isomer of the lactic acid is:
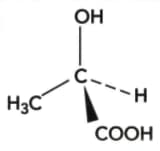
Draw the other optical isomer.
A sample of lactic acid, , was extracted from a natural source and found to be optically active. Explain why lactic acid scan form optical isomers.
A sample of lactic acid, , was extracted from a natural source and found to be optically active. It was then subjected to two reactions, as shown below.
Sample was optically active but sample was not optically active.
Give the reagents and conditions necessary for step A.
A sample of lactic acid, , was extracted from a natural source and found to be optically active. It was then subjected to two reactions, as shown below.
Sample was optically active but sample was not optically active.
Give the balance equation for the reaction in step A.
A sample of lactic acid, , was extracted from a natural source and found to be optically active. It was then subjected to two reactions, as shown below.
Sample was optically active but sample was not optically active.
Give the reagents and conditions necessary for step B.
