Diagram shows an isochore, an isotherm, an adiabatic and two isobars of two gases on a work done versus heat supplied curve. The initial states of both gases are the same and the scales for the two axes are same.
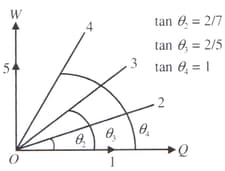
Which of the following statements is incorrect?

Important Questions on Thermodynamics
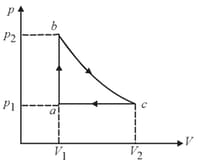
Carbon monoxide is carried around a closed cycle in which is an isothermal process as shown in the figure. The gas absorbs of heat as its temperature increases from to in going from to . The quantity of heat rejected by the gas during the process is approximately
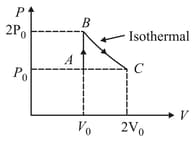
Three processes compose a thermodynamic cycle shown in the accompanying diagram. Process takes place at constant temperature, process takes place at constant volume and process is adiabatic. During the complete cycle the total amount of work done is . During process of work is done on the system. Which of the following statements is incorrect?
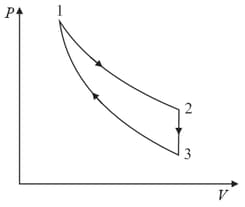
Find work done by the gas in the process shown in figure.
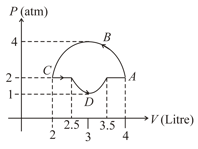
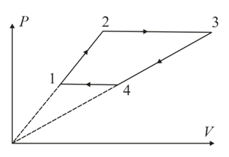
Three moles of an ideal monoatomic gas perform a cyclic process as shown in the figure. The gas temperature in different states are and Find the work done during the cycle.