MEDIUM
AMU-AT (B.Tech.)
IMPORTANT
Earn 100
Different curves in the figure show the behaviour of gases
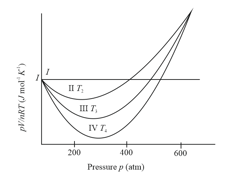
(i) Curve I represent ideal gas behaviour
(ii) Curves II, III and IV also represents ideal gas behaviour at different temperatures and
(iii) Curves II, III and IV represents behaviour of a real gas at different temperatures and
(iv)
(v)
The correct statements are

(ii) Curves II, III and IV also represents ideal gas behaviour at different temperatures and
(iii) Curves II, III and IV represents behaviour of a real gas at different temperatures and
(iv)
(v)
The correct statements are
(a)(i), (ii), (iv)
(b)(i), (iii), (iv)
(c)(i), (iii), (v)
(d)(i), (ii), (v)
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
AMU-AT (B.Tech.)
IMPORTANT
Two non-reactive monatomic ideal gases have their atomic masses in the ratio . The ratio of their partial pressures, when enclosed in a vessel kept at a constant temperature is . The ratio of their densities is
