Differentiate between: Distillation and Fractional distillation.
Important Questions on Is Matter around Us Pure?
Given below are two statements:
Statement-I: Retardation factor can be measured in meter/centimetre.
Statement-II: value of a compound remains constant in all solvents.
Choose the most appropriate answer from the options given below:
Using the provided information in the following paper chromatogram:
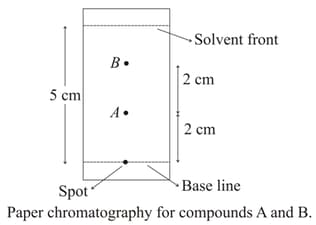
The calculated value of .
(
)
At a refinery, petroleum is separated into several components by a process called fractional distillation using a fractionating column. Which of the following statement is incorrect about the process?
(I) Temperature decreases from the bottom to the top of the column
(II) At each level in the column only one compound is collected
(III) The fraction collecting at the top of the column is less volatile
(IV) The fraction with the highest boiling point condenses first and gets collected near the base of the fractionating column.
Give an example for the mixture having the following characteristics. Suggest a suitable method to separate the components of the mixture.
Two volatile components with appreciable difference in boiling points.

