Differentiate between adsorption and absorption.
Important Questions on Adsorption and Colloids
Given:
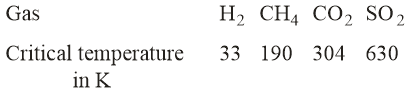
On the basis of data given above, predict which of the following gases shows the least adsorption on a definite amount of charcoal?
Define the following term : Adsorbent
Write any two differences between Absorption and Adsorption.
of is adsorbed on of platinum powder at and bar pressure. The volume of the gas adsorbed per gram of the adsorbent is
(Given : bar )
Write three differences between adsorption and absorption.
of oxygen is adsorbed on of platinum metal. The volume of oxygen adsorbed per gram of the adsorbent at and in is
One gram of activated charcoal has total surface area equal to 103 m2. If radius of a gaseous molecule is 10-8 cm and the gas shows only monolayer adsorption on the surface of charcoal, then volume of gas at STP adsorbed on total surface of g charcoal is.
of acetic acid is shaken with activated charcoal. The concentration of acetic acid is reduced to of original molarity. The weight of acetic acid adsorbed per of charcoal is
An equal volume of gases and are adsorbed by one gram of charcoal at in the order:

