MEDIUM
Earn 100
Differentiate between the reversible and irreversible process on the basis of entropy change.
Important Questions on Thermodynamics
MEDIUM

MEDIUM
EASY
(Latent heat of ice is and )
HARD

The correct option(s) is (are)
EASY
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




HARD
One mole of an ideal monoatomic gas undergoes two reversible processes and as shown in the given figure:
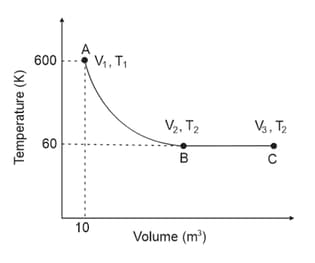
is an adiabatic process. If the total heat absorbed in the entire process and is , the value of is
[Use molar heat capacity of the gas at constant pressure, ]
EASY
EASY
Reversible process
HARD
HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
HARD
MEDIUM
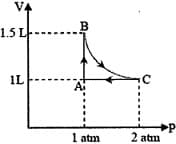
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
EASY
MEDIUM
HARD
(: pressure, : volume, : temperature, : enthalpy, : entropy)
EASY

